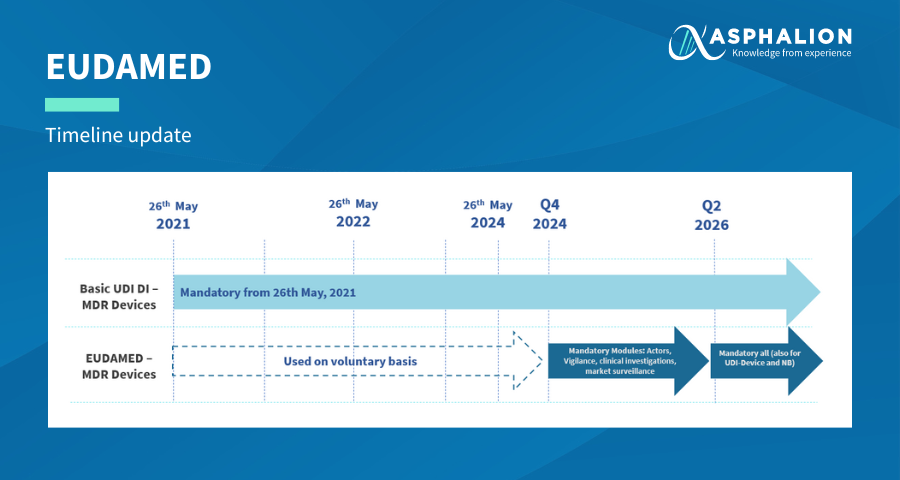
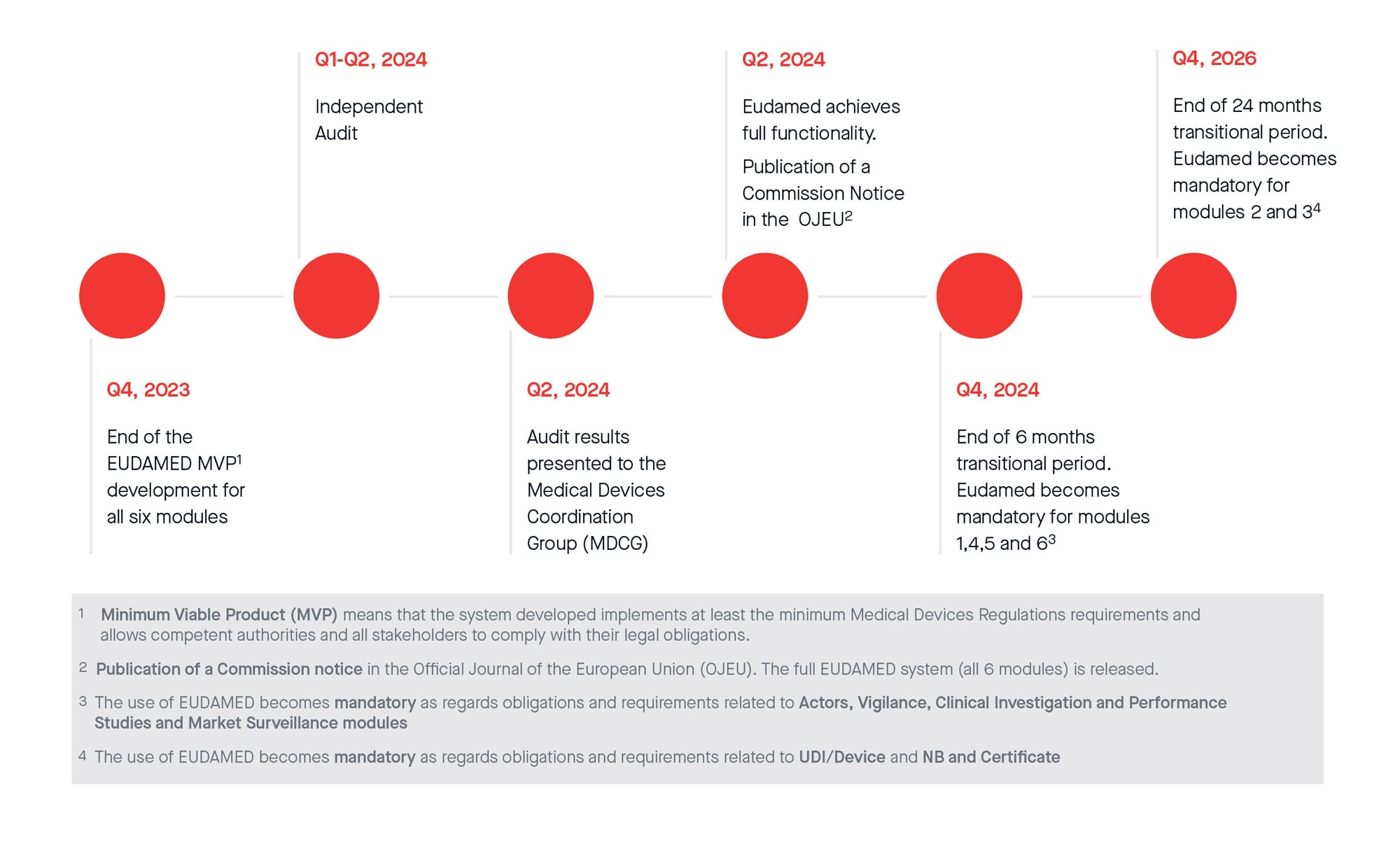
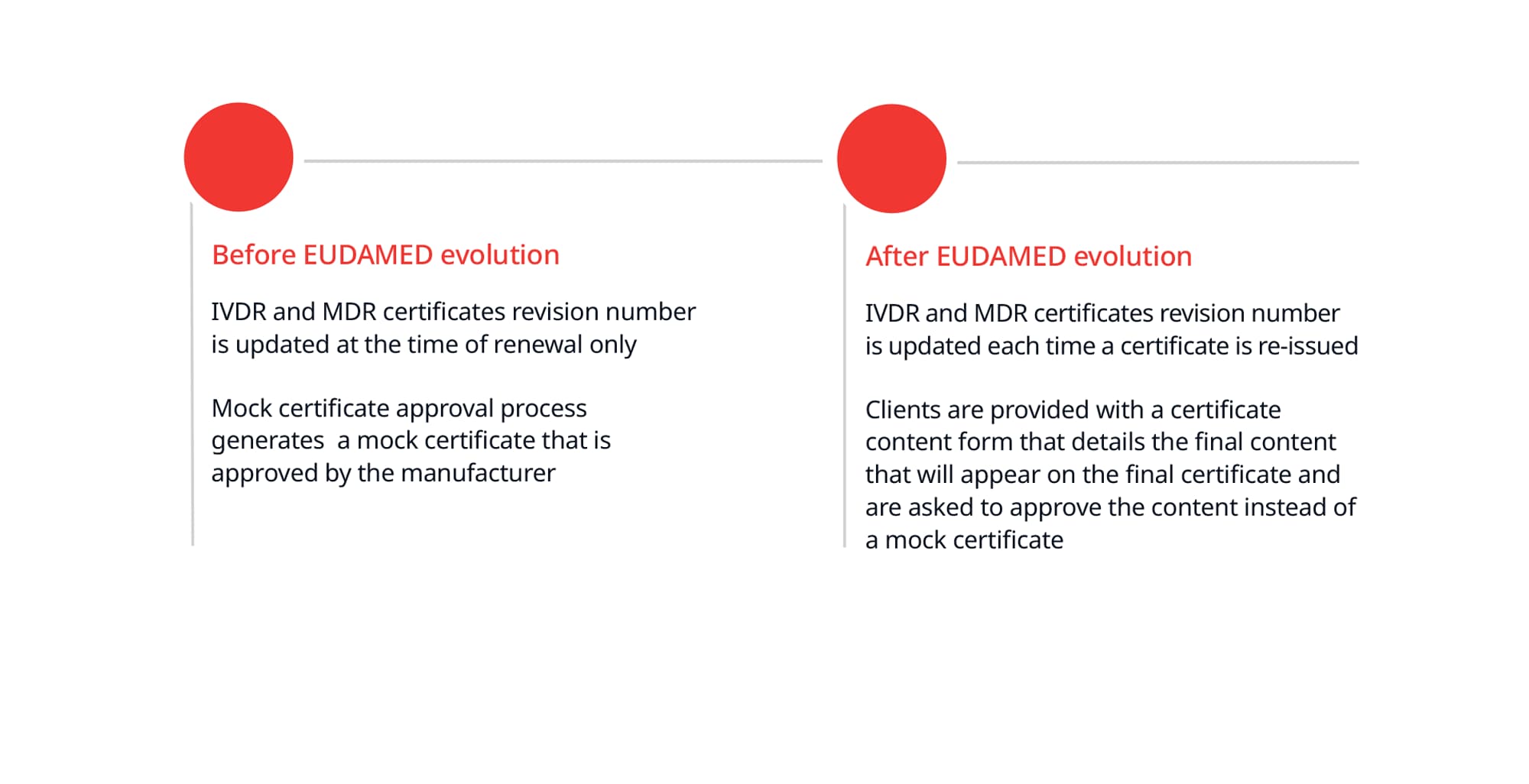
New European medical device regulation: How the French ecosystem should seize the opportunity of the EUDAMED and the UDI system, while overcoming the constraints thereof - ScienceDirect

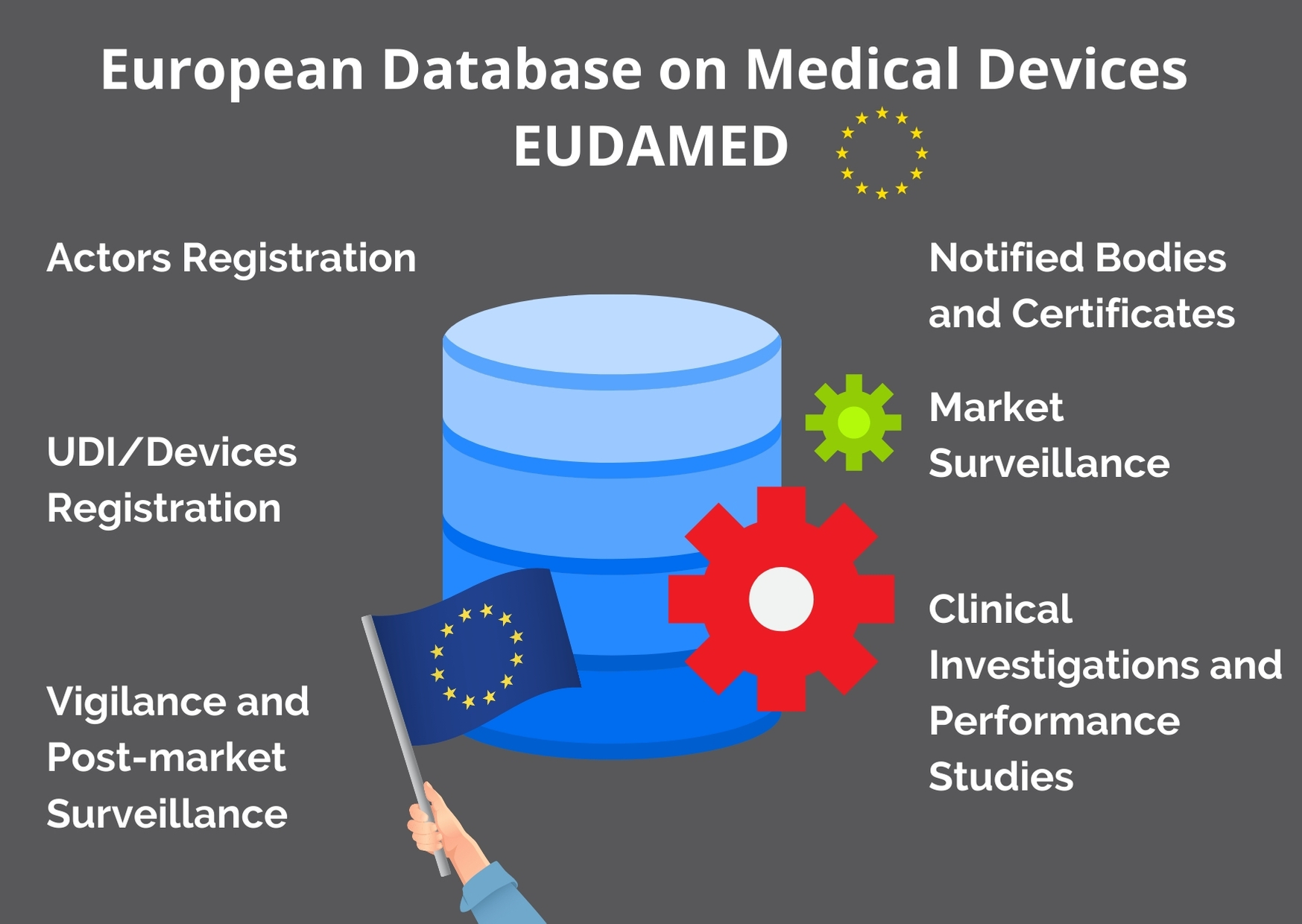
UDI & EUDAMED Explained under EU MDR - Clinical, Regulatory & Automation solutions for Life Sciences
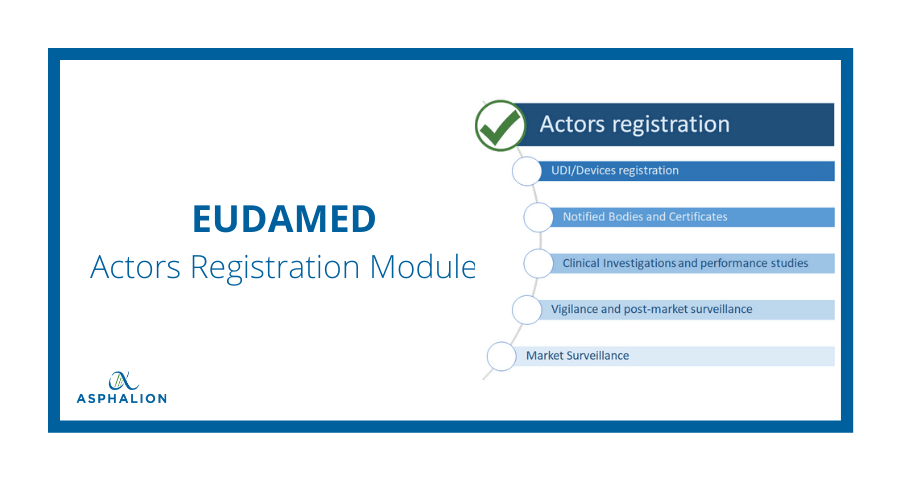
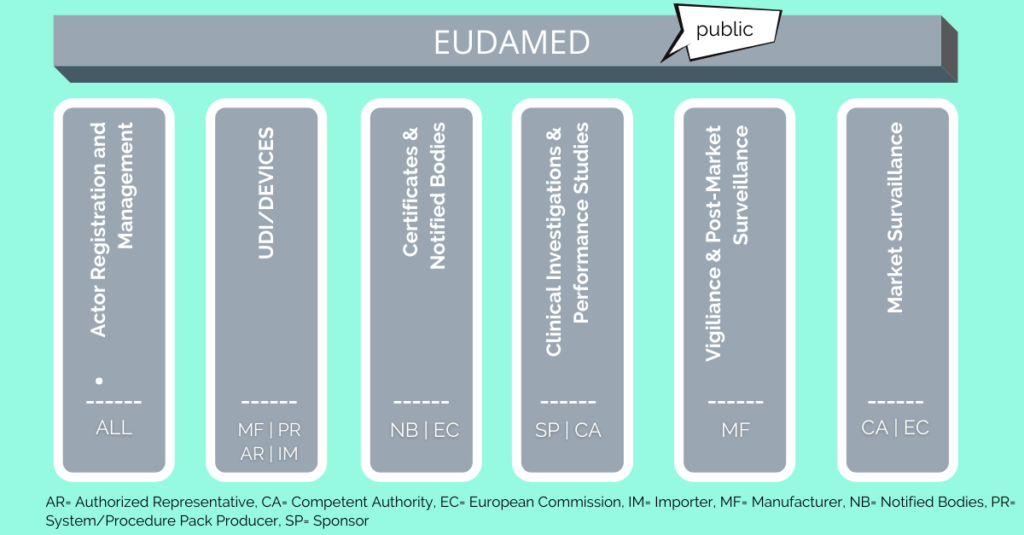
![Blog] EUDAMED – the key role of each module - Pharma Serialization, Aggregation and Track and Trace Software by SoftGroup Blog] EUDAMED – the key role of each module - Pharma Serialization, Aggregation and Track and Trace Software by SoftGroup](https://softgroup.eu/wp-content/uploads/2021/06/EUDAMED-2.png)