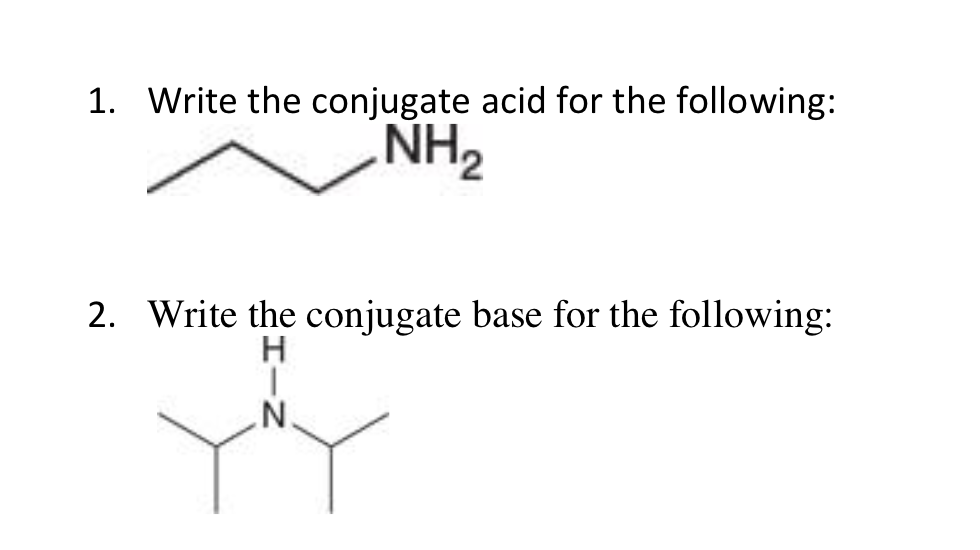
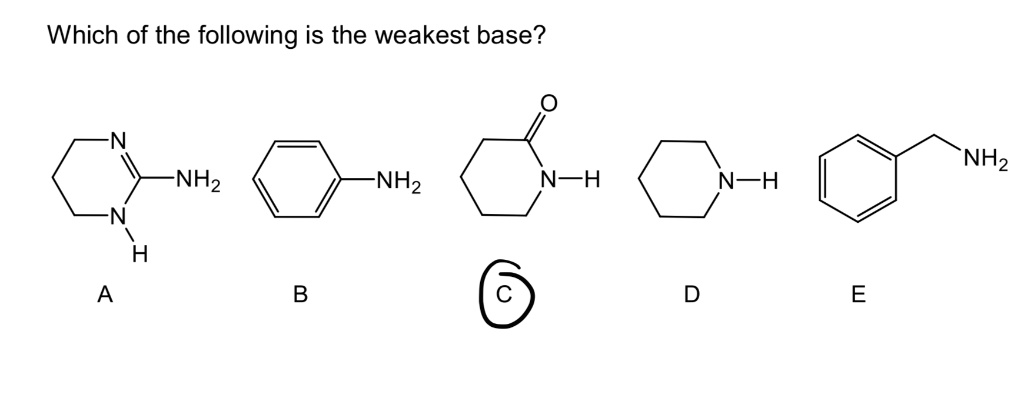
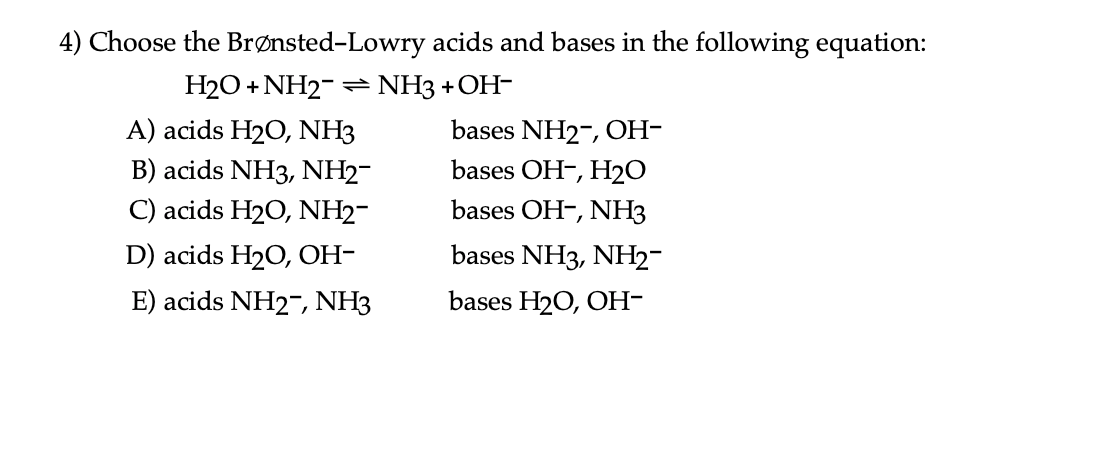
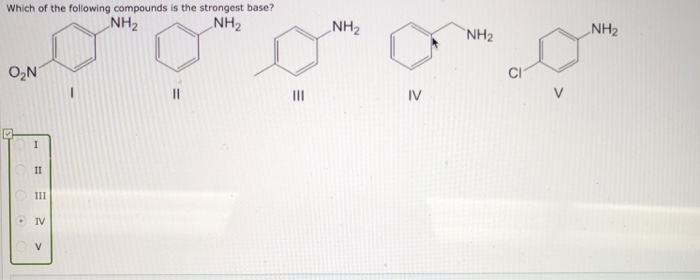
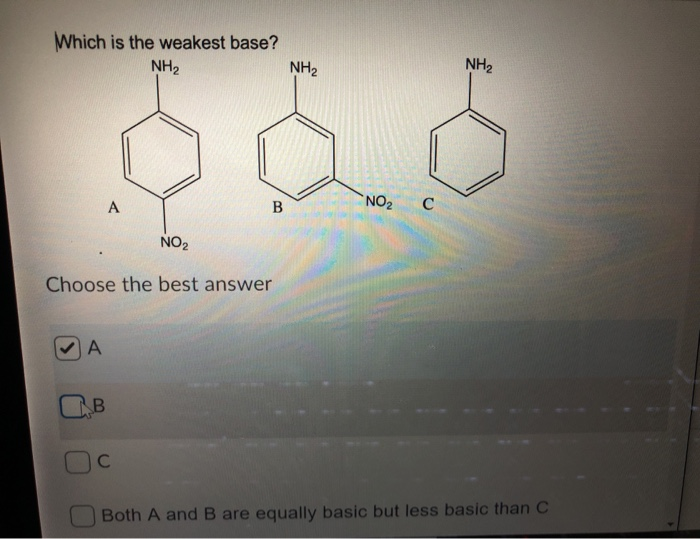
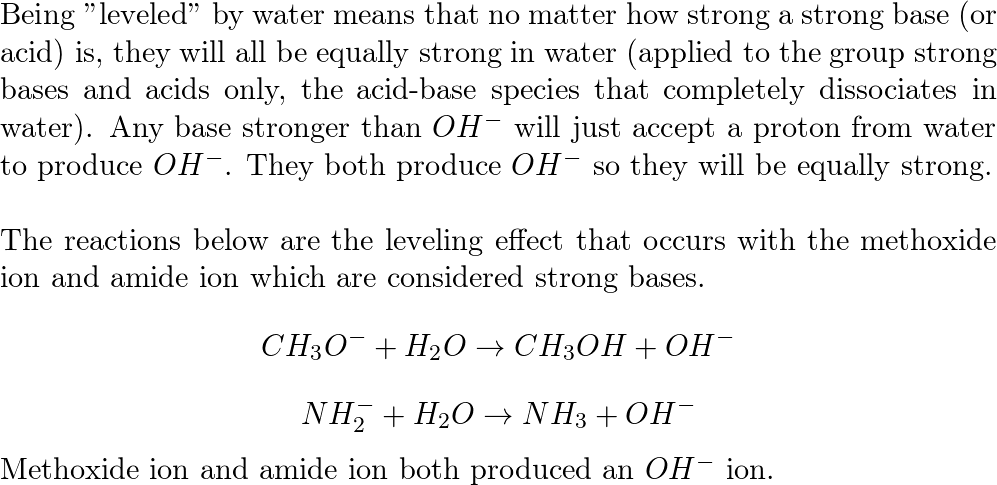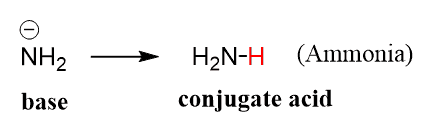![CH3 - OH|CH - CH2 - CH2 - NH2 [ Triethylamine ]Ethyl formate Product The major product of the given reaction is: CH3 - OH|CH - CH2 - CH2 - NH2 [ Triethylamine ]Ethyl formate Product The major product of the given reaction is:](https://dwes9vv9u0550.cloudfront.net/images/7494062/22483b28-e987-494e-85b6-898b531ad092.jpg)
CH3 - OH|CH - CH2 - CH2 - NH2 [ Triethylamine ]Ethyl formate Product The major product of the given reaction is:

Tröger's Base Polyimide Hybrid Membranes by Incorporating UiO-66-NH2 Nanoparticles for Gas Separation | Industrial & Engineering Chemistry Research

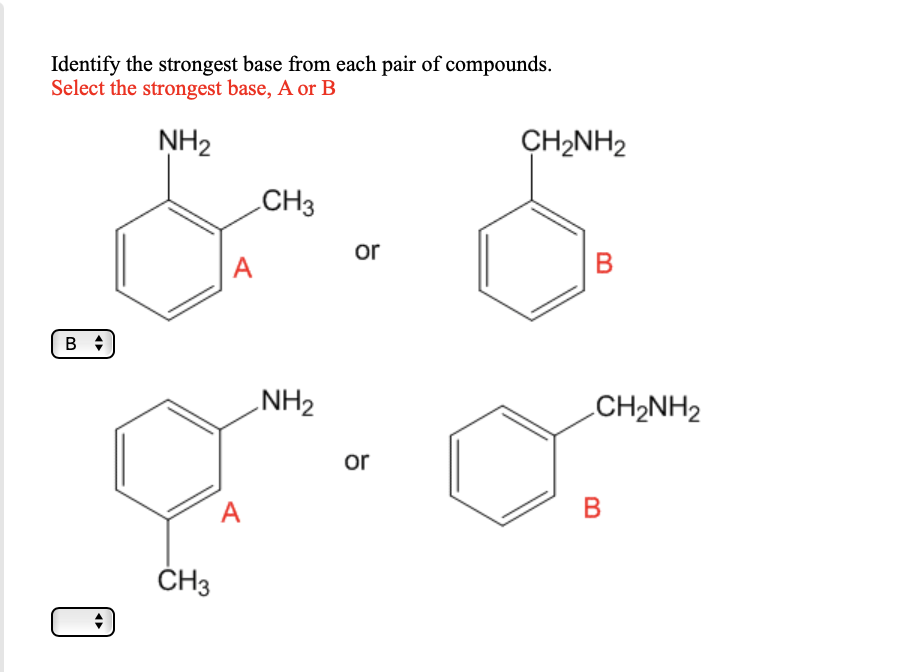
OneClass: NH2 b. 1-(amino meth 㠗ょOH - 3. Select the stronger base from each pair of compounds Cl ...

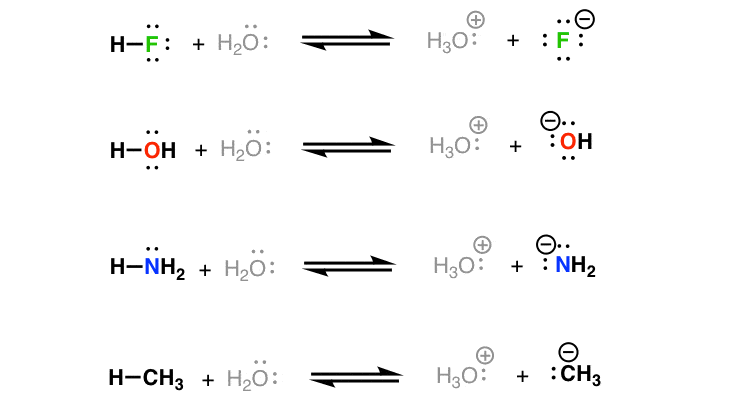
What is the decreasing order of strength of the following bases: OH-, NH2-, H-C=C-, CH3-CH2- ? What's the explanation? - Quora
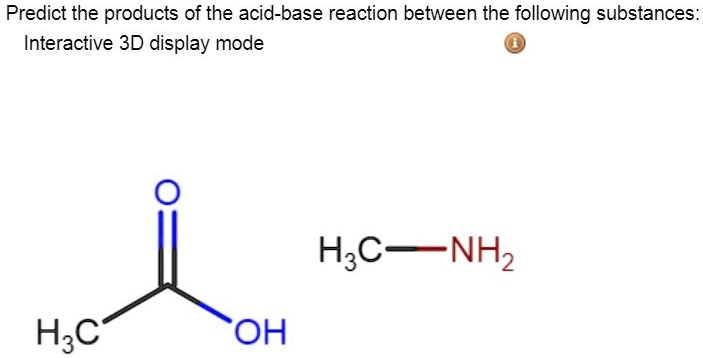
SOLVED: Predict the products of the acid-base reaction between the following substances: Interactive 3D display mode H3C NH2 H3C - OH
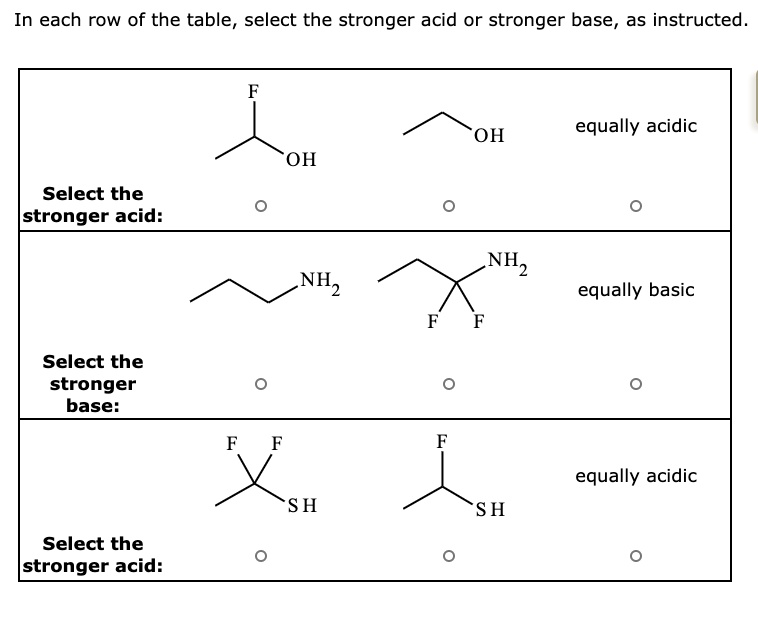
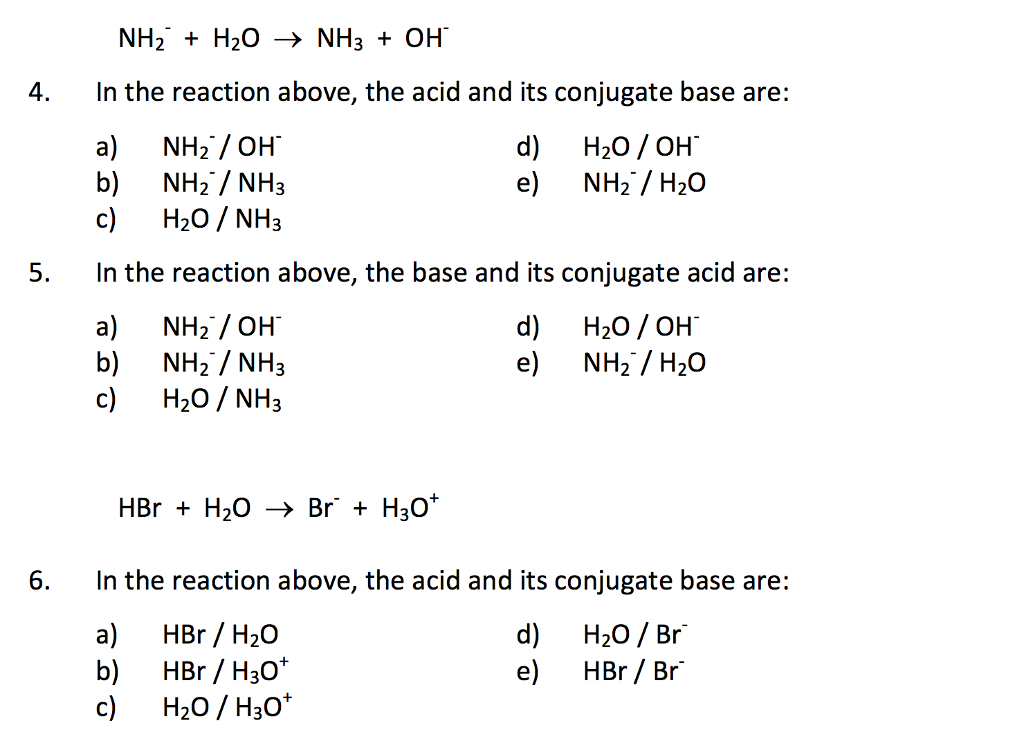
SOLVED: In each row of the table, select the stronger acid or stronger base, as instructed. equally acidic OH OH Select the Istronger acid: NH equally basic Select the stronger base: equally