Effect of Solvent on the Lithium−Bromine Exchange of Aryl Bromides: Reactions of n-Butyllithium and tert-Butyllithium with 1-Bromo-4-tert-butylbenzene at 0 °C | The Journal of Organic Chemistry
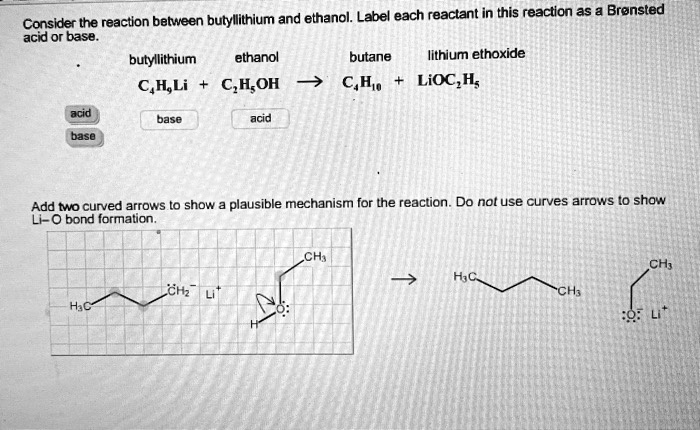
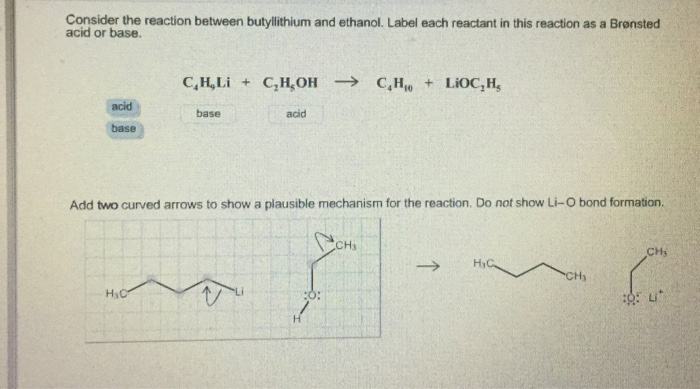
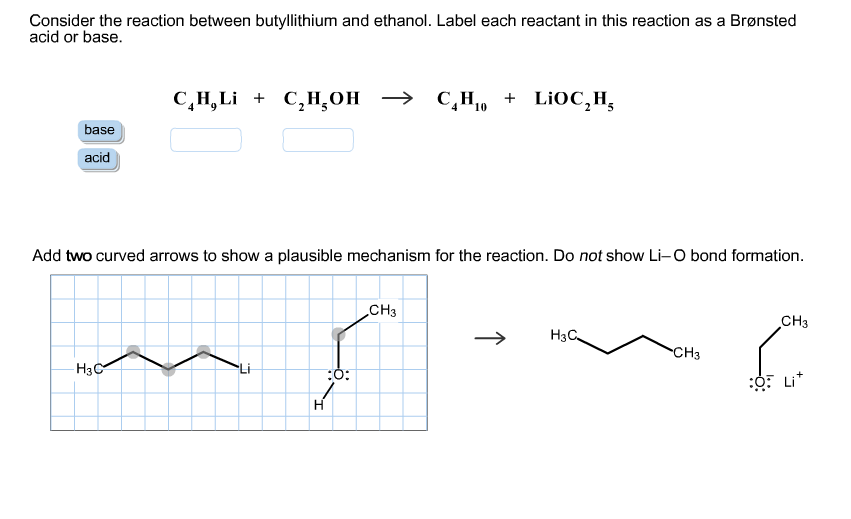
SOLVED: reaction belween butylithium and ethanol: Label each reactant in this reaction as Bronsted Consider the acid or base. butyllithium ethanol butane Ilthium ethoxide CH,Li C,H;OH C,H,o LiOC,Hs acid base acid base
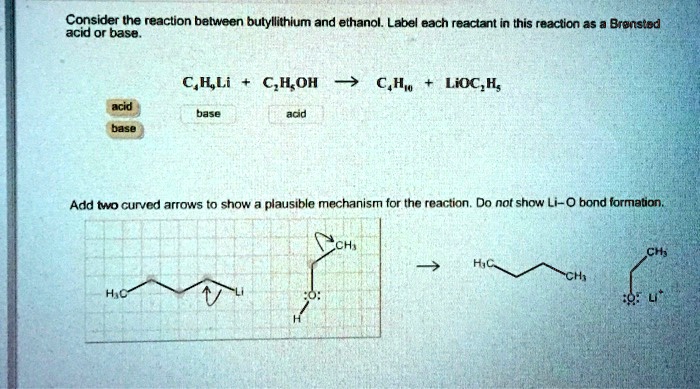
SOLVED: Consider the reaclion batween butyllithium and ethanol Labee each reactant in this reaciion as a Brensted acid Or base C,HLi C,HOH LiOc,H; acid Dase add Dasa Add two curved Altows t0

n-Butyllithium/N,N,N',N'-Tetramethylethylenediamine-Mediated Ortholithiations of Aryl Oxazolines: Substrate-Dependent Mechanisms | Journal of the American Chemical Society
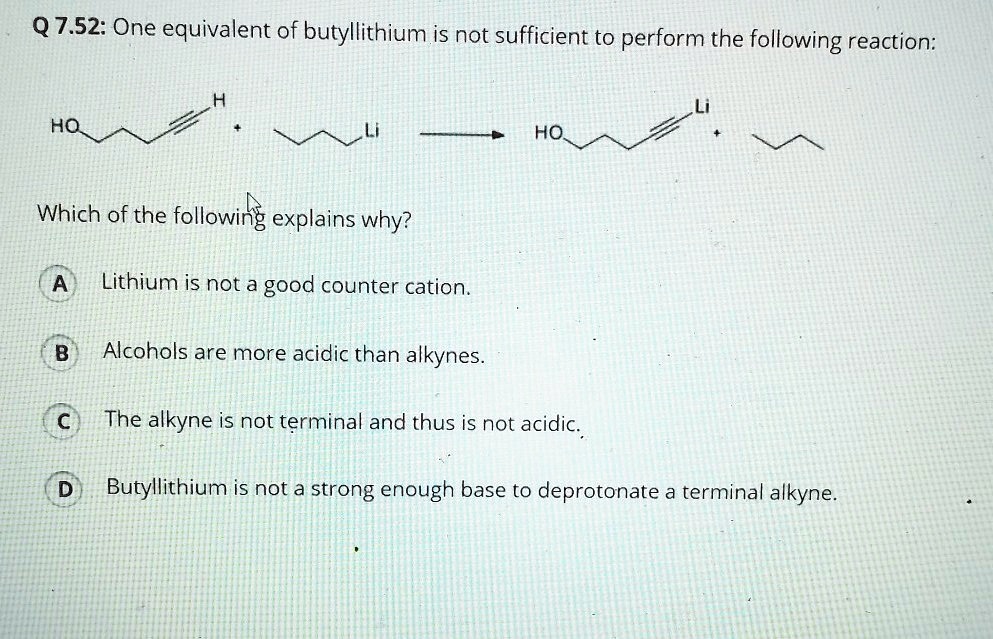
SOLVED: Q7.52: One equivalent of butyllithium is not sufficient to perform the following reaction: HO HO Which of the following explains why? Lithium is not a good counter cation. Alcohols are more

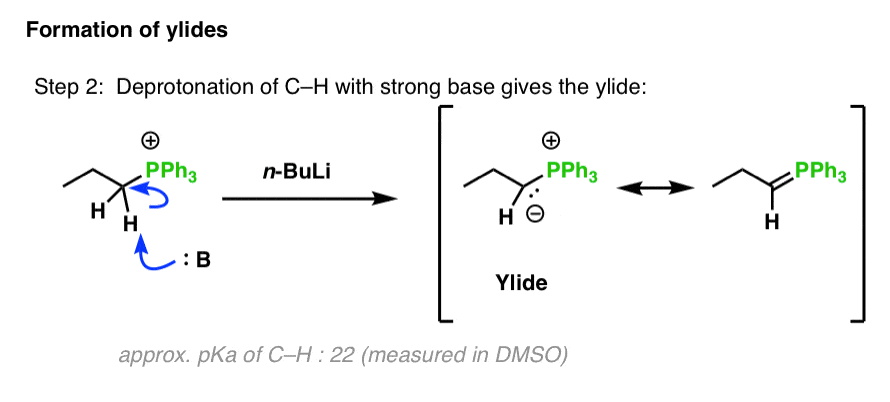
SOLVED: Match the species below to their appropriate, relative basic strength. butyllithium acetate alkoxide acetate Weakest Base alkoxide 2) Medium strength base 3) Strongest Base butyllithium

Acros Organics AC187540090 sec-Butyllithium, 1.3M solution in cyclohexane/hexane (92/8) (9g) from Cole-Parmer India

n-Butyllithium-promoted regioselective elimination of vicinal bis-triflate having an adjacent ether oxygen - ScienceDirect