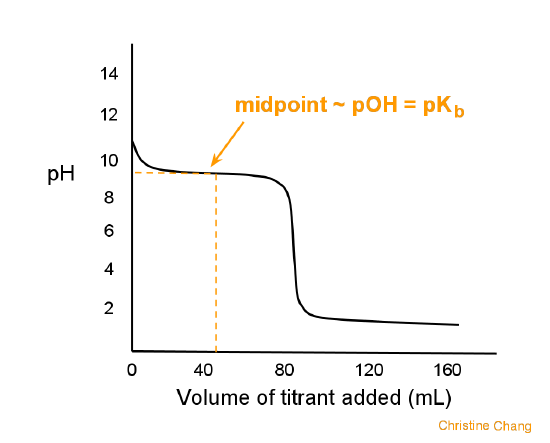
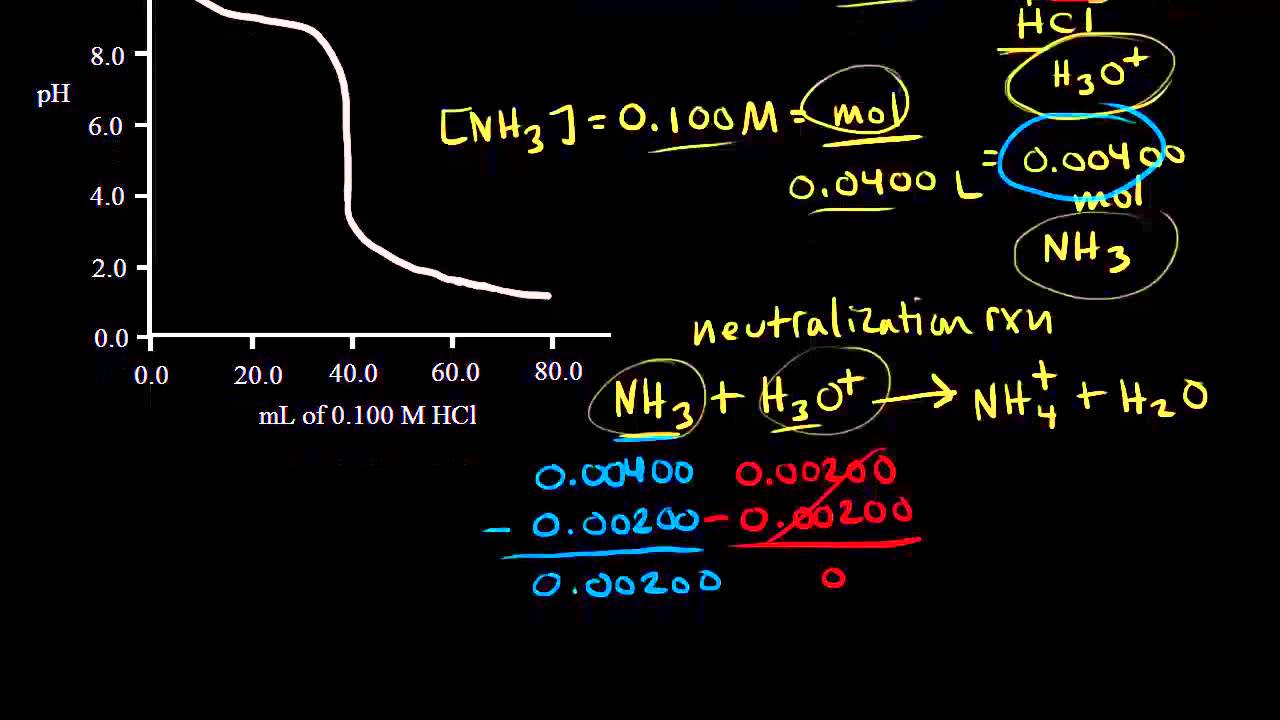
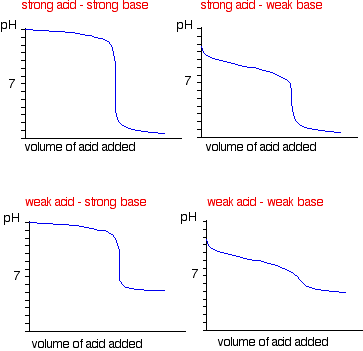
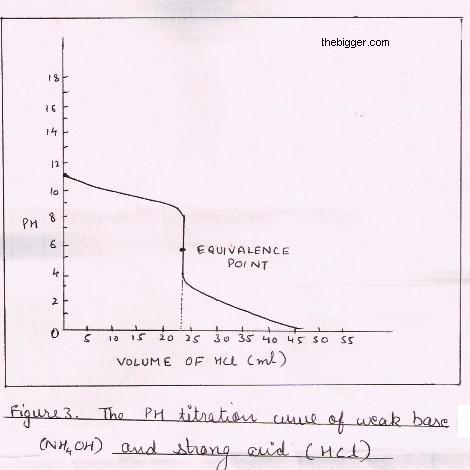
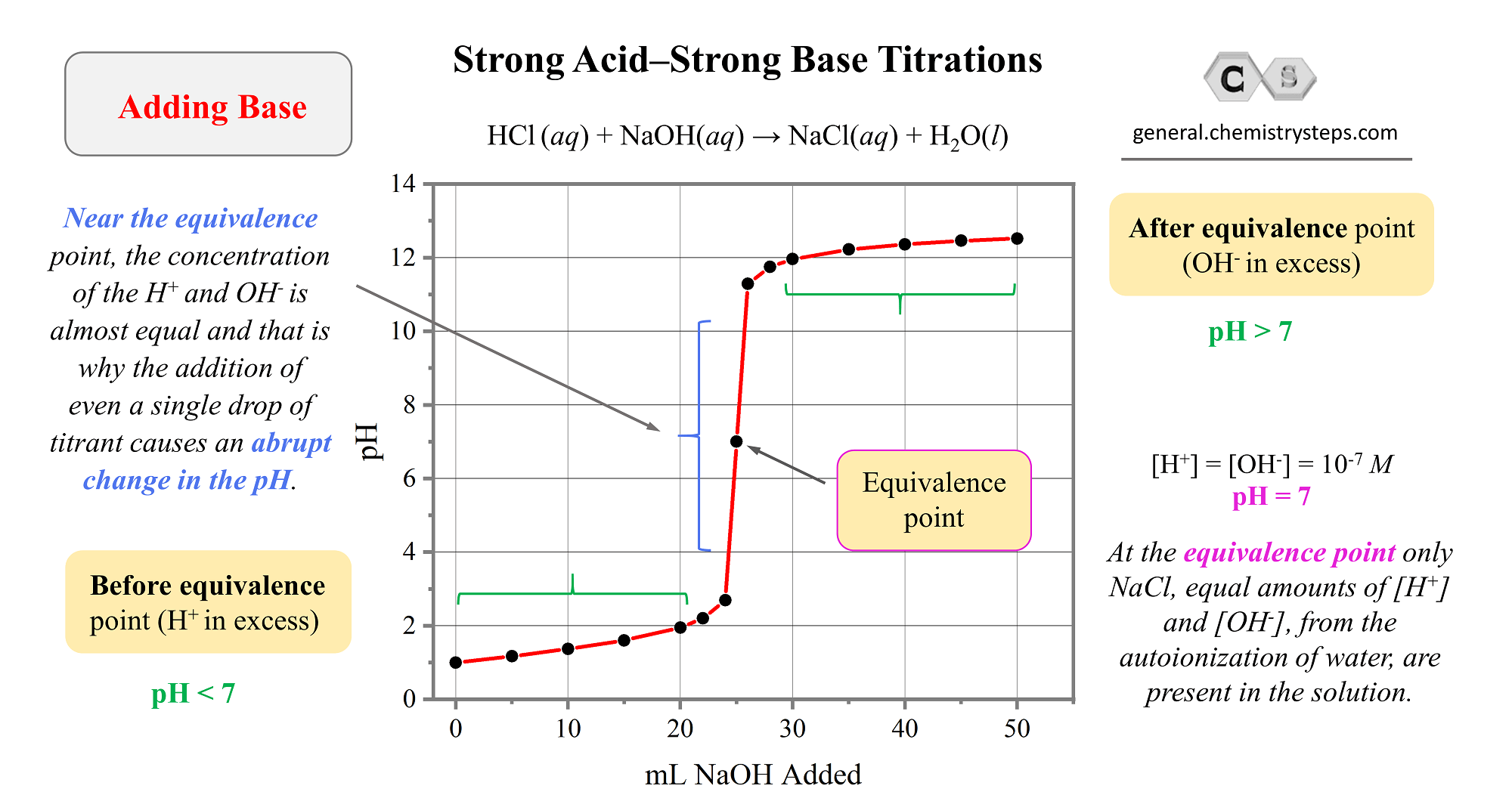
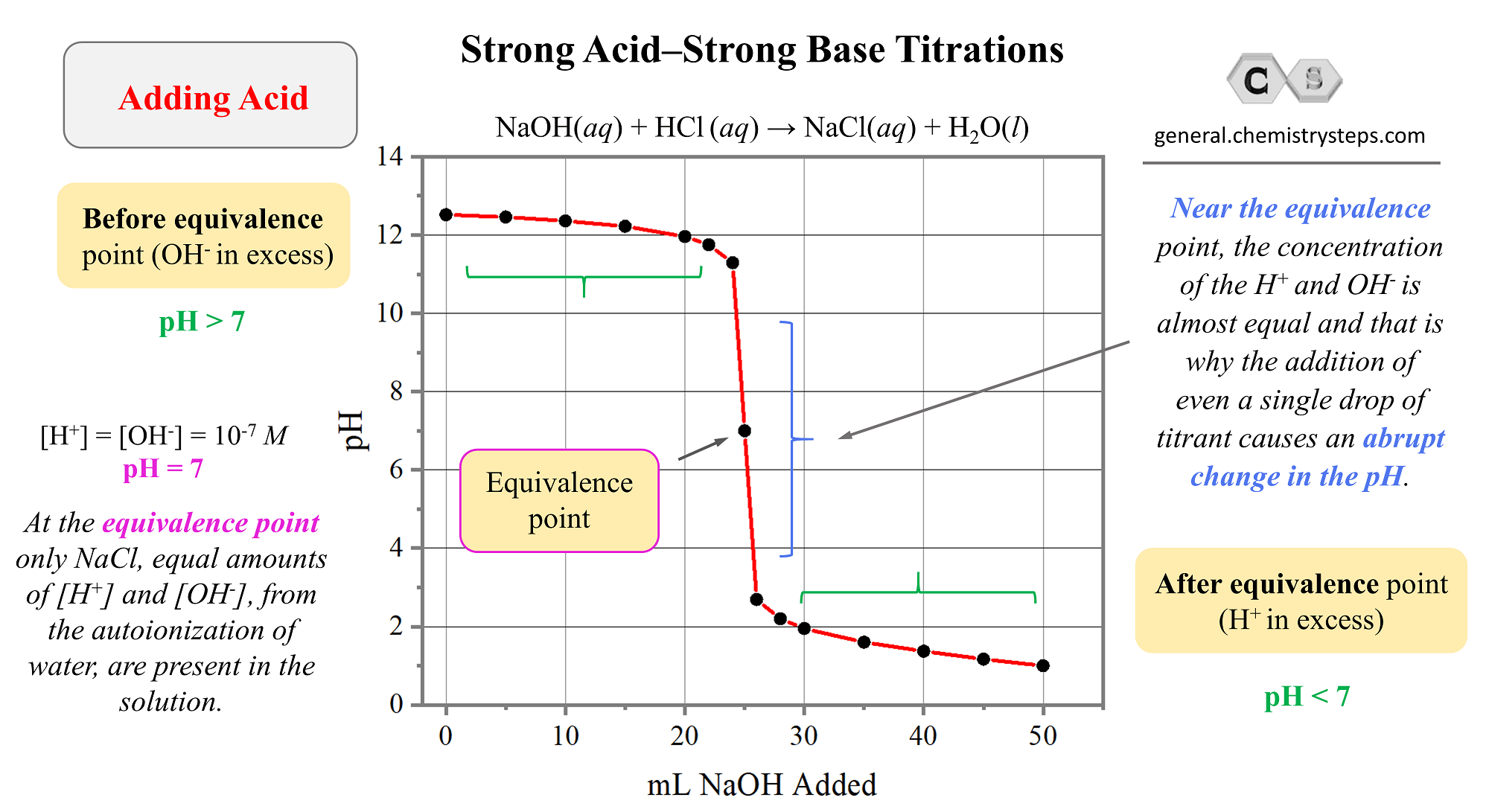
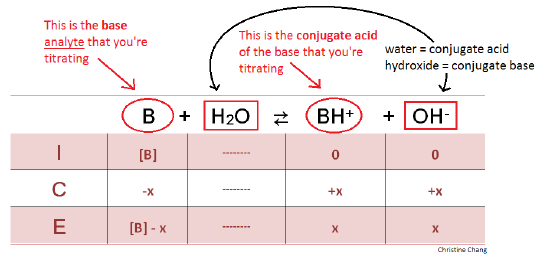
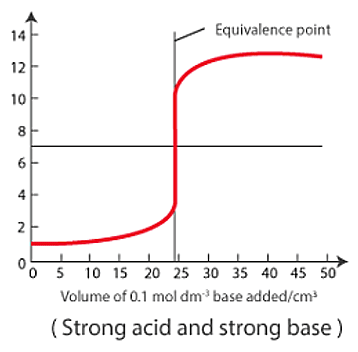
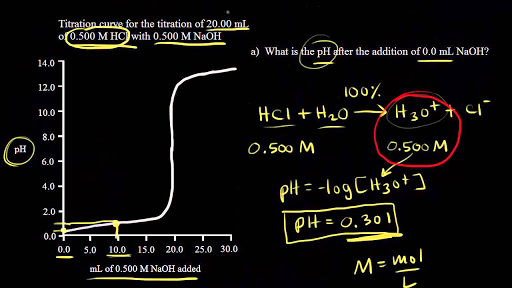
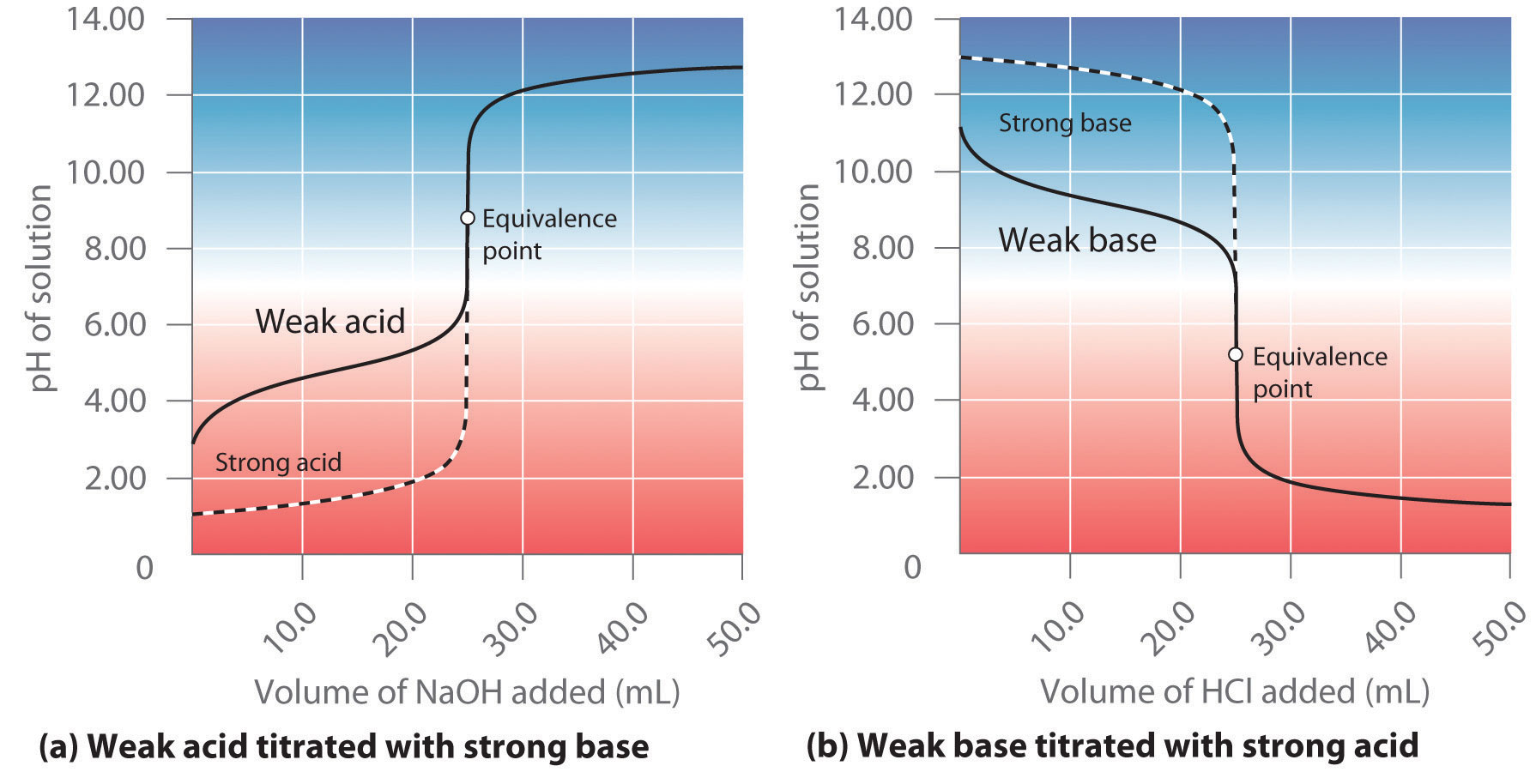
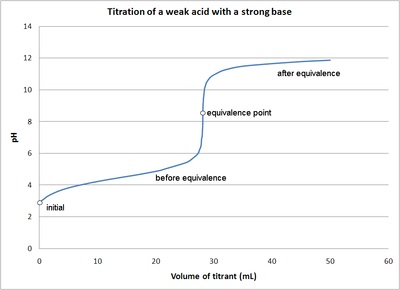
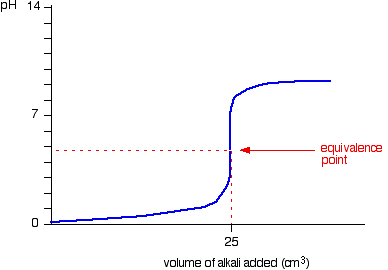
Sketch the following titration curves. a) A strong acid/strong base. b) A weak monoprotic acid/strong base. c) A weak diprotic acid/strong base. | Homework.Study.com

Why is phenolphthalein an appropriate indicator for titration of a strong acid with a strong base? - Chemistry Stack Exchange
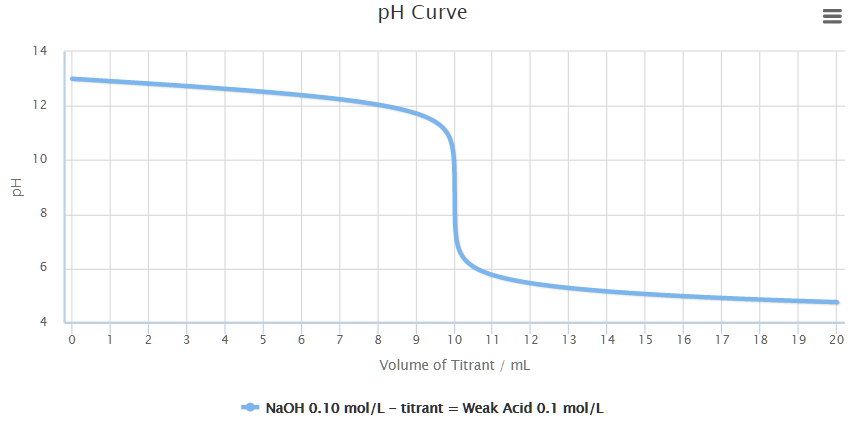
While performing the titration of a weak acid and strong base, can we put weak acid in the strong base rather than the usual strong acid in a weak base? | Socratic

Strong Acid–Strong Base Titration Curve | Image and Video Exchange ForumImage and Video Exchange Forum

Conductometric titration of weak acid and weak base (weak acid vs weak base)/Conductometry - YouTube

Conductometric titration of strong acid and weak base (strong acid vs weak base)/Conductometry - YouTube