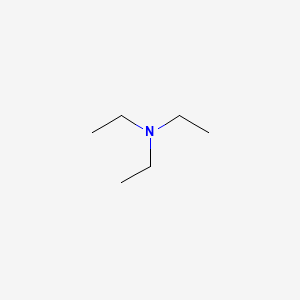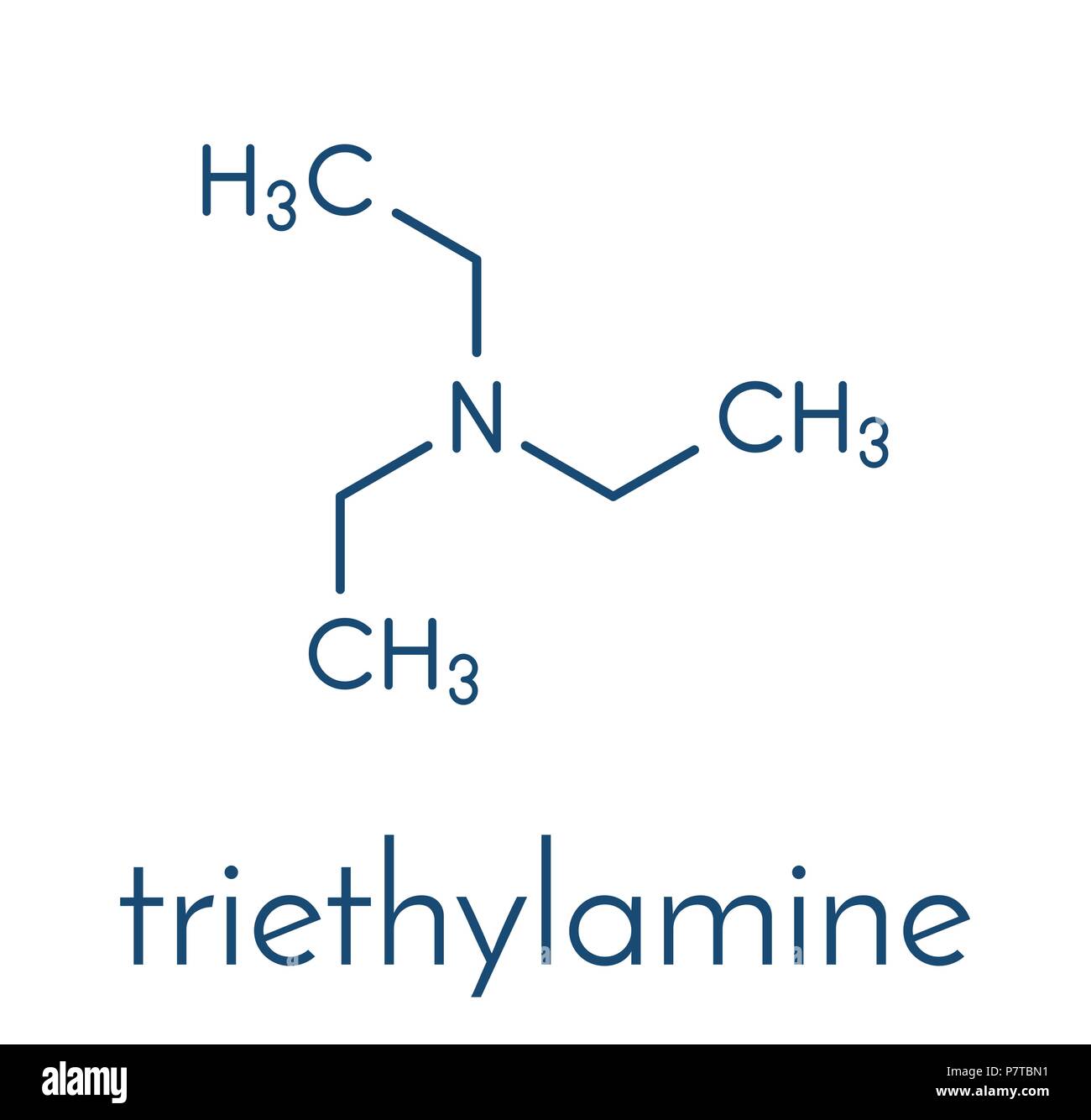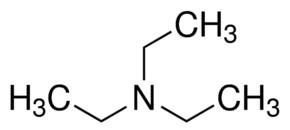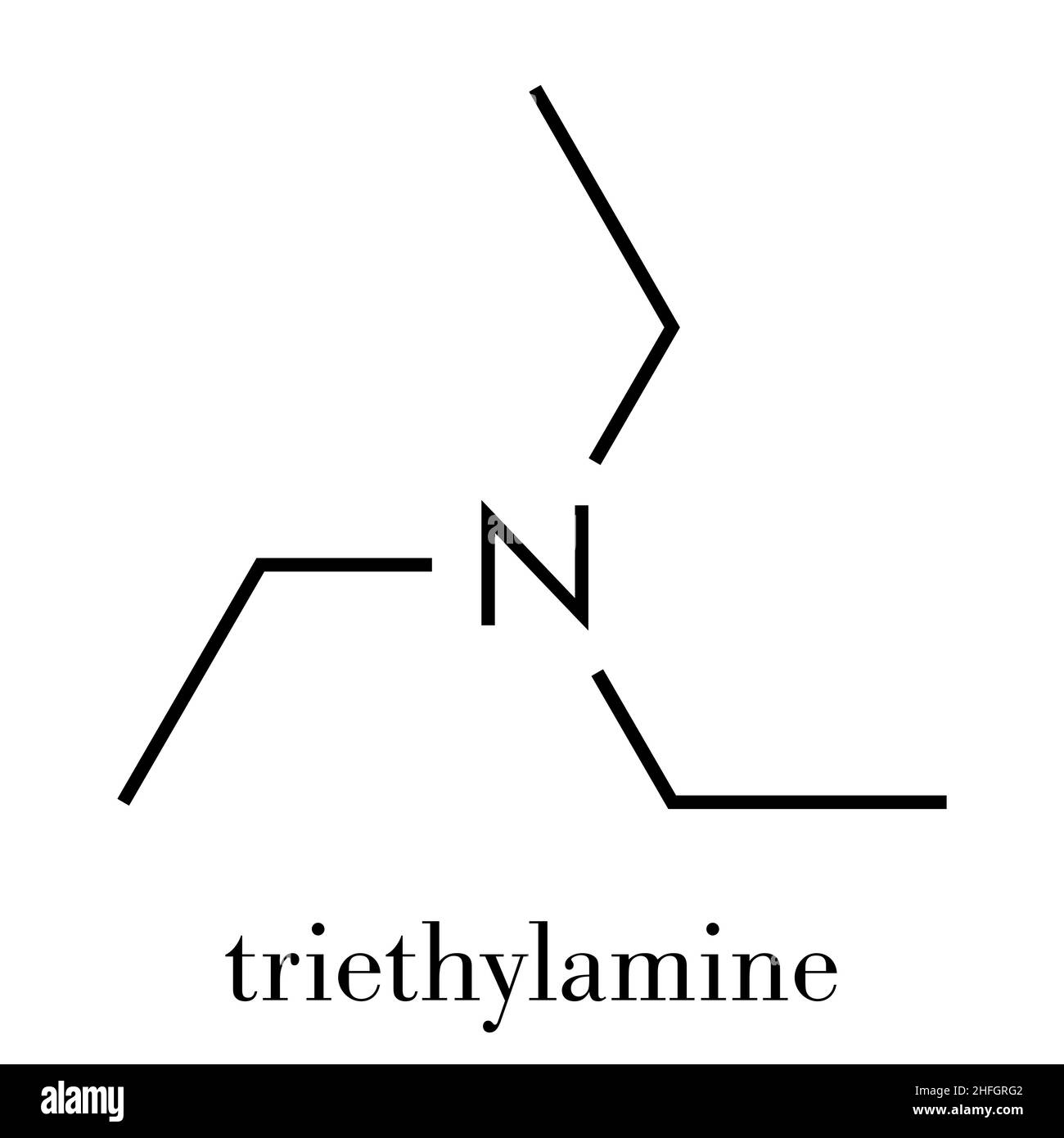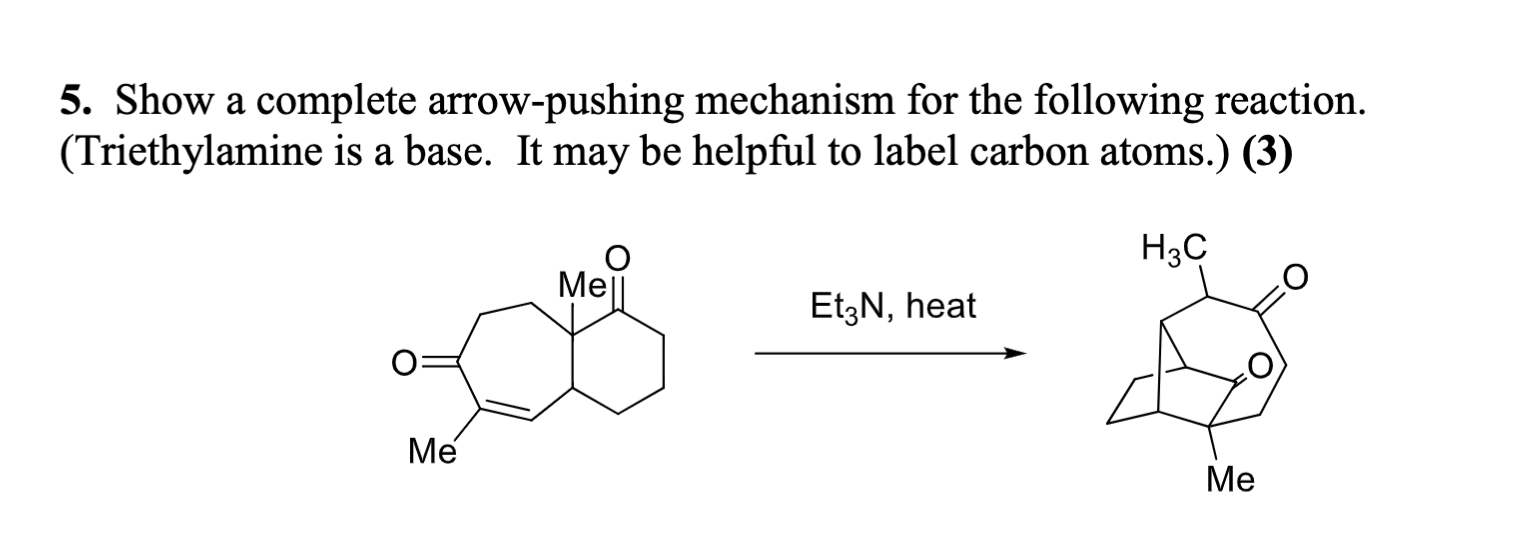
Triethylamine: a potential N-base surrogate for pyridine in Knoevenagel condensation of aromatic aldehydes and malonic acid - New Journal of Chemistry (RSC Publishing)
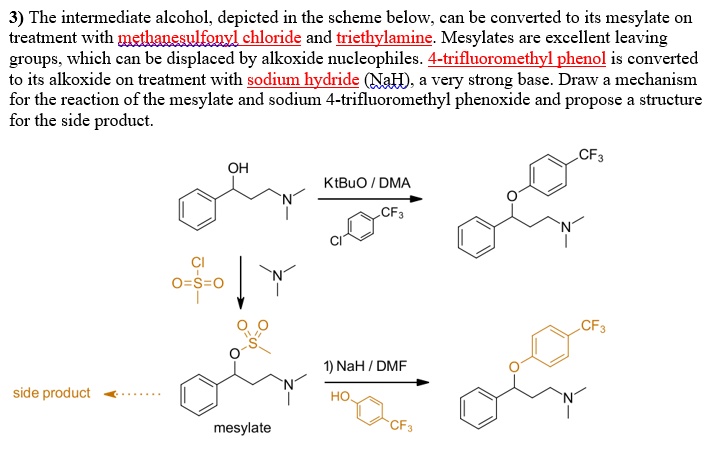
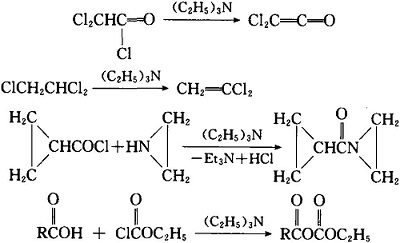
SOLVED: 3) The intermediate alcohol, depicted in the scheme below; can be converted to its mesylate on treatment with methavesulfonvi chloride and triethylamine: Mesylates are excellent leaving groups which can be displaced

PDF) Triethylamine: an efficient N-base catalyst for synthesis of annulated uracil derivativies in aqueous ethanol
Triethylamine organic base molecule. 3D rendering. Atoms are represented as spheres with conventional color coding: hydrogen (white), carbon (grey), n Stock Photo - Alamy
Triethylamine Organic Base Molecule. 3D Rendering Stock Illustration - Illustration of chemical, amine: 186807292

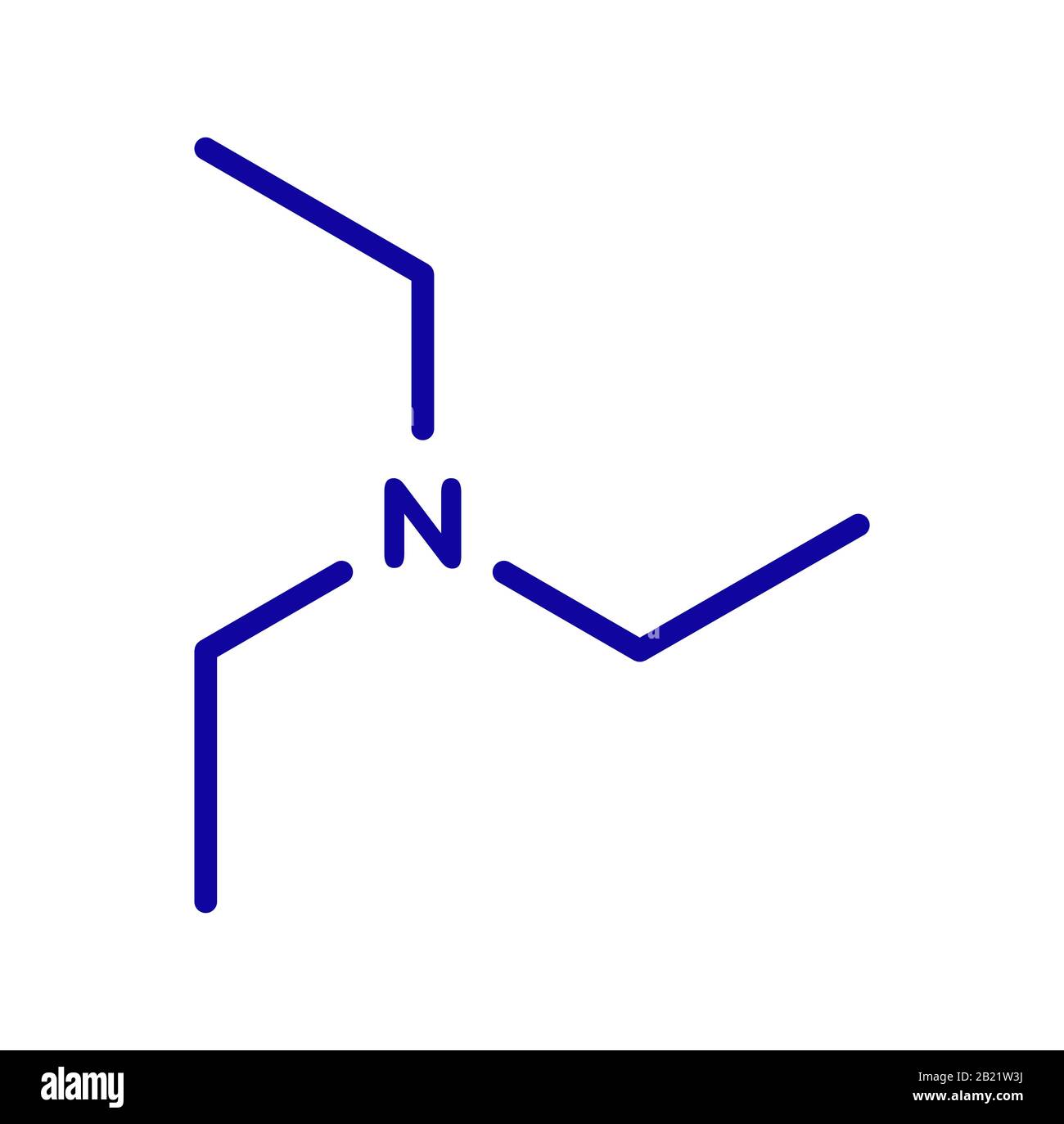
Triethylamine Organic Base Molecule Skeletal Formula Stock Vector (Royalty Free) 1128981863 | Shutterstock
Triethylamine: a potential N-base surrogate for pyridine in Knoevenagel condensation of aromatic aldehydes and malonic acid - New Journal of Chemistry (RSC Publishing) DOI:10.1039/C5NJ03125G